Table of Contents
Atmosphere was coined by 17th-century scientists combining the Greek words for vapor, atmos, and sphere, sphaira. The earth’s atmosphere is the air and gas surrounding our planet. Atmosphere also describes the feeling of a place. A coffee shop might have a cool, artsy atmosphere. Some plants grow best in a damp atmosphere. In physics, atmosphere is a unit of pressure equal to the air pressure at sea level.
Atmosphere, the gas and aerosol envelope that extends from the ocean, land, and ice-covered surface of a planet outward into space. The density of the atmosphere decreases outward, because the gravitational attraction of the planet, which pulls the gases and aerosols (microscopic suspended particles of dust, soot, smoke, or chemicals) inward, is greatest close to the surface.
Earth’s atmosphere has been able to contain water in each of its three phases (solid, liquid, and gas), which has been essential for the development of life on the planet.
Gases in Earth’s Atmosphere :
Nitrogen and oxygen are by far the most common; dry air is composed of about 78% nitrogen (N2) and about 21% oxygen (O2). Argon, carbon dioxide (CO2), and many other gases are also present in much lower amounts; each makes up less than 1% of the atmosphere’s mixture of gases. The atmosphere also includes water vapor. The amount of water vapor present varies a lot, but on average is around 1%. There are also many small particles – solids and liquids – “floating” in the atmosphere. These particles, which scientists call “aerosols”, include dust, spores and pollen, salt from sea spray, volcanic ash, smoke, and more.
Radiation :
The temperature of the atmosphere and surface is influenced by electromagnetic radiation, and this radiation is traditionally divided into two types: insolation from the Sun and emittance from the surface and the atmosphere. Insolation is frequently referred to as shortwave radiation; it falls primarily within the ultraviolet and visible portions of the electromagnetic spectrum and consists predominantly of wavelengths of 0.39 to 0.76 micrometers (0.00002 to 0.00003 inch). Radiation emitted from Earth is called longwave radiation; it falls within the infrared portion of the spectrum and has typical wavelengths of 4 to 30 micrometers (0.0002 to 0.001 inch). Wavelengths of radiation emitted by a body depend on the temperature of the body, as specified by Planck’s radiation law. The Sun, with its surface temperature of around 6,000 kelvins (K; about 5,725 °C, or 10,337 °F), emits at a much shorter wavelength than does Earth, which has lower surface and atmospheric temperature.
Layers Of Earth’s Atmosphere :
Earth’s atmosphere has a series of layers, each with its own specific traits. Moving upward from ground level, these layers are named the troposphere, stratosphere, mesosphere, thermosphere and exosphere. The exosphere gradually fades away into the realm of interplanetary space.
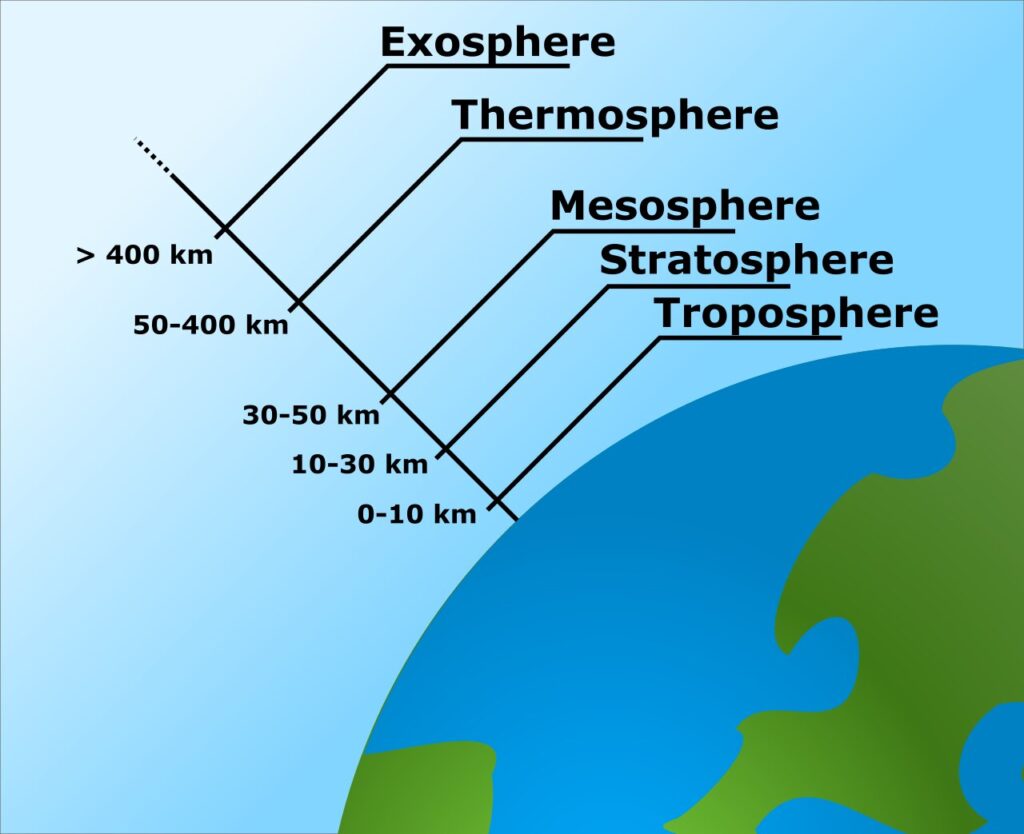
Troposphere :
This is the lowest part of the atmosphere – the part we live in. It contains most of our weather – clouds, rain, snow. In this part of the atmosphere the temperature gets colder as the distance above the earth increases, by about 6.5°C per kilometer. The actual change of temperature with height varies from day to day, depending on the weather.
The troposphere contains about 75% of all of the air in the atmosphere, and almost all of the water vapour (which forms clouds and rain). The decrease in temperature with height is a result of the decreasing pressure. If a parcel of air moves upwards it expands (because of the lower pressure). When air expands it cools. So air higher up is cooler than air lower down.
The lowest part of the troposphere is called the boundary layer. This is where the air motion is determined by the properties of the Earth’s surface. Turbulence is generated as the wind blows over the Earth’s surface, and by thermals rising from the land as it is heated by the sun. This turbulence redistributes heat and moisture within the boundary layer, as well as pollutants and other constituents of the atmosphere.
The top of the troposphere is called the tropopause. This is lowest at the poles, where it is about 7 – 10 km above the Earth’s surface. It is highest (about 17 – 18 km) near the equator.
Stratosphere :
Located between approximately 12 and 50 kilometers (7.5 and 31 miles) above Earth’s surface, the stratosphere is perhaps best known as home to Earth’s ozone layer, which protects us from the Sun’s harmful ultraviolet radiation. Because of that UV radiation, the higher up you go into the stratosphere, the warmer temperatures become. The stratosphere is nearly cloud- and weather-free, but polar stratospheric clouds are sometimes present in its lowest, coldest altitudes. It’s also the highest part of the atmosphere that jet planes can reach.
Mesosphere :
Above the stratosphere is the mesosphere. It extends upward to a height of about 85 km (53 miles) above our planet. Most meteors burn up in the mesosphere. Unlike the stratosphere, temperatures once again grow colder as you rise up through the mesosphere. The coldest temperatures in Earth’s atmosphere, about -90° C (-130° F), are found near the top of this layer. The air in the mesosphere is far too thin to breathe; air pressure at the bottom of the layer is well below 1% of the pressure at sea level, and continues dropping as you go higher.
Thermosphere :
Located between about 80 and 700 kilometers (50 and 440 miles) above Earth’s surface is the thermosphere, whose lowest part contains the ionosphere. In this layer, temperatures increase with altitude due to the very low density of molecules found here. It is both cloud- and water vapor-free. The aurora borealis and aurora australis are sometimes seen here. The International Space Station orbits in the thermosphere.
Exosphere :
The exosphere is the outermost layer of Earth’s atmosphere (i.e. the upper limit of the atmosphere). It extends from the exobase, which is located at the top of the thermosphere at an altitude of about 700 km above sea level, to about 10,000 km (6,200 mi; 33,000,000 ft) where it merges into the solar wind.
This layer is mainly composed of extremely low densities of hydrogen, helium and several heavier molecules including nitrogen, oxygen and carbon dioxide closer to the exobase. The atoms and molecules are so far apart that they can travel hundreds of kilometers without colliding with one another. Thus, the exosphere no longer behaves like a gas, and the particles constantly escape into space. These free-moving particles follow ballistic trajectories and may migrate in and out of the magnetosphere or the solar wind.
The exosphere is located too far above Earth for any meteorological phenomena to be possible. However, the aurora borealis and aurora Australis sometimes occur in the lower part of the exosphere, where they overlap into the thermosphere. The exosphere contains many of the satellites orbiting Earth.
Temperature :
Temperature trends in two thick layers of the atmosphere as measured between January 1979 and December 2005 by Microwave Sounding Units and Advanced Microwave Sounding Units on NOAA weather satellites. The instruments record microwaves emitted from oxygen molecules in the atmosphere.
The division of the atmosphere into layers mostly by reference to temperature is discussed above. Temperature decreases with altitude starting at sea level, but variations in this trend begin above 11 km, where the temperature stabilizes through a large vertical distance through the rest of the troposphere. In the stratosphere, starting above about 20 km, the temperature increases with height, due to heating within the ozone layer caused by the capture of significant ultraviolet radiation from the Sun by the dioxygen and ozone gas in this region. Still another region of increasing temperature with altitude occurs at very high altitudes, in the aptly-named thermosphere above 90 km.
The Ozone Layer :
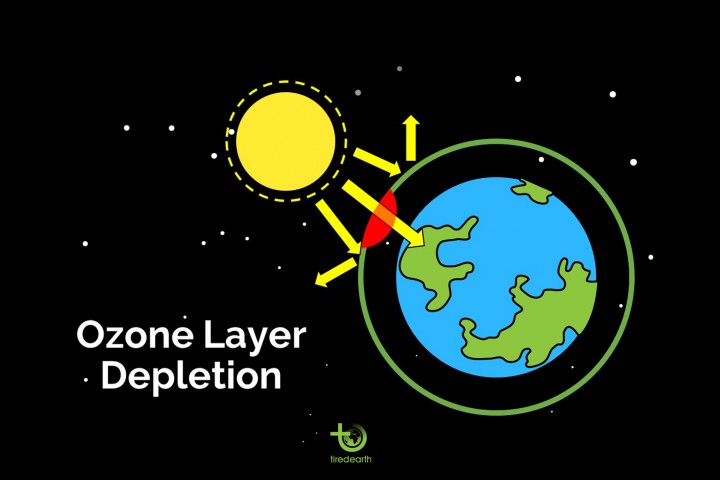
The Earth’s atmosphere is composed of several layers. The lowest ,troposphere extends from the Earth’s surface up to about 6 miles or 10 kilometers (km) in altitude. Virtually all human activities occur in the troposphere.
The ozone layer in the stratosphere absorbs a portion of the radiation from the sun, preventing it from reaching the planet’s surface. Most importantly, it absorbs the portion of UV light called UVB. UVB has been linked to many harmful effects, including skin cancers, cataracts, and harm to some crops and marine life.
World Ozone Day :
This Day is observed on September 16 every year to spread awareness among people about the depletion of Ozone Layer. This year the slogan for World Ozone Day is ‘Ozone For Life’.
Ozone Layer Depletion :
Ozone layer depletion is the gradual thinning of the earth’s ozone layer present in the upper atmosphere. Ozone depletion also consists of a much larger springtime decrease in stratospheric ozone around Earth’s polar regions, which is referred to as the ozone hole.
The main cause of ozone depletion and the ozone hole is manufactured chemicals, especially manufactured halocarbon refrigerants, solvents, propellants, and foam- blowing agents (chlorofluorocarbons (CFCs), HCFCs, halons). Since the early 1970’s, scientists observed reduction in stratospheric ozone and it was found more prominent in Polar Regions. ODS substances have a lifetime of about 100 years.
Global warming is caused primarily by putting too much carbon dioxide into the atmosphere when coal, oil, and natural gas are burned to generate electricity or to run our cars. Carbon dioxide spreads around the planet like a blanket, and is one of the main gases responsible for the absorption of infrared radiation (felt as heat), which comprises the bulk of solar energy.
The Ozone Hole :
The term ‘ozone hole’ refers to the depletion of the protective ozone layer in the upper atmosphere (stratosphere) over Earth’s polar regions. People, plants, and animals living under the ozone hole are harmed by the solar radiation now reaching the Earth’s surface—where it causes health problems, from eye damage to skin cancer.
Stratospheric ozone is constantly produced by the action of the sun’s ultraviolet radiation on oxygen molecules (known as photochemical reactions). Although ozone is created primarily at tropical latitudes, large-scale air circulation patterns in the lower stratosphere move ozone toward the poles, where its concentration builds up.
In addition to this global motion, strong winter polar vortices are also important to concentrating ozone at the poles. During the continuously dark polar winter, the air inside the polar vortices becomes extremely cold, a necessary condition for polar stratospheric cloud formation. Polar stratospheric clouds create the conditions for drastic ozone destruction, providing a surface for chlorine to change into ozone-destroying form. They generally last until the sun comes up in the spring.

